Trends in precision medicine are enabling treatments tailored to individual genetic profiles and lifestyles. Advances in artificial intelligence (AI), genomics, and data analytics are driving this shift and improving treatment efficacy and patient outcomes.
The global precision medicine market size is predicted to reach around USD 470.53 billion by 2034. The market size is improving at a compound annual growth rate (CAGR) of 16.50% from 2025 to 2034. This report will benefit industry leaders, innovation managers, and corporations seeking to stay ahead in personalized healthcare by understanding the latest precision medicine developments and their strategic implications.
What are the Top 10 Trends in Precision Medicine in 2025?
- AI Integration
- Precision Oncology
- Personalized Diagnostics
- Drug Discovery Technologies
- Biomarkers
- Cell and Gene Therapies (CGTs)
- Multi-Omics
- Microbiome
- Clinical Decision Support Systems (CDSS)
- Ethical AI
Methodology: How We Created the Precision Medicine Trend Report
For our trend reports, we leverage our proprietary StartUs Insights Discovery Platform, covering 7M+ global startups, 20K technologies & trends, plus 150M+ patents, news articles, and market reports.
Creating a report involves approximately 40 hours of analysis. We evaluate our own startup data and complement these insights with external research, including industry reports, news articles, and market analyses. This process enables us to identify the most impactful and innovative trends in the precision medicine industry.
For each trend, we select two exemplary startups that meet the following criteria:
- Relevance: Their product, technology, or solution aligns with the trend.
- Founding Year: Established between 2020 and 2025.
- Company Size: A maximum of 200 employees.
- Location: Specific geographic considerations.
This approach ensures our reports provide reliable, actionable insights into the precision medicine innovation ecosystem while highlighting startups driving technological advancements in the industry.
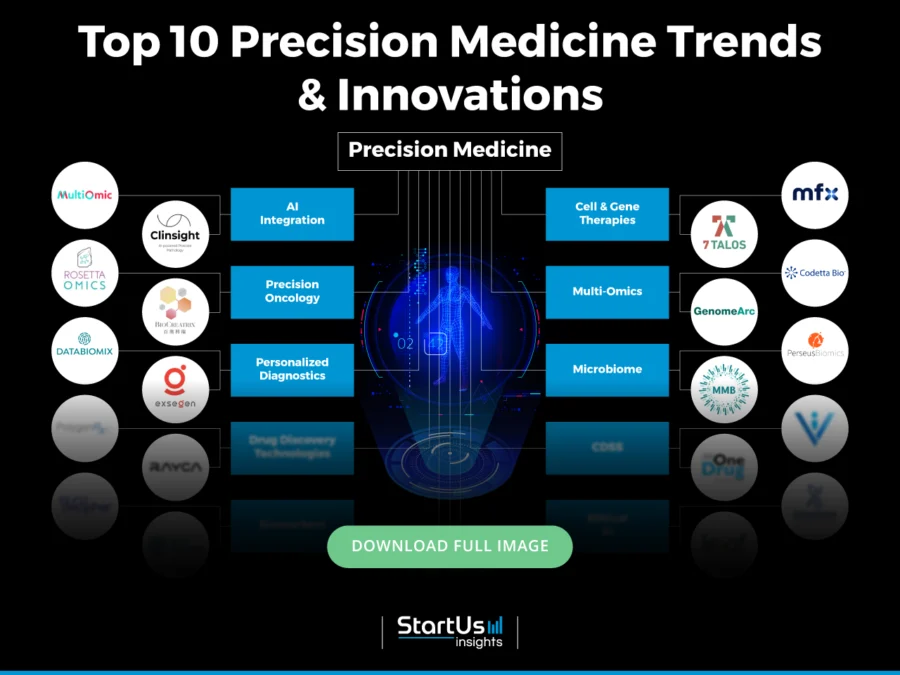
Innovation Map outlines the Top 10 Trends in Precision Medicine & 20 Promising Startups
For this in-depth research on the Top Precision Medicine Trends & Startups, we analyzed a sample of 1268 global startups & scaleups. The Precision Medicine Innovation Map, created from this data-driven research, helps you improve strategic decision-making by giving you a comprehensive overview of the precision medicine industry trends & startups that impact your company.
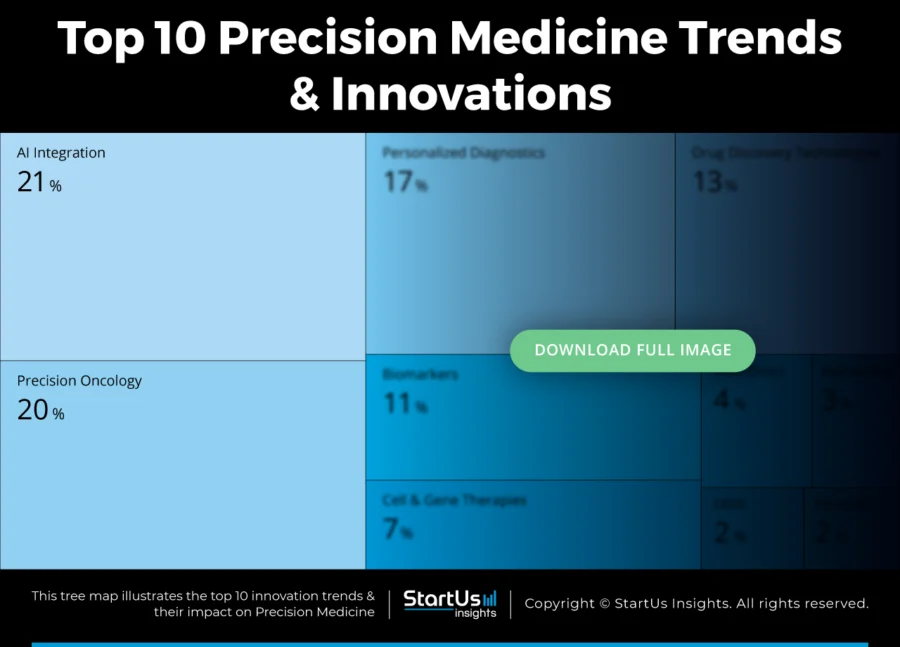
Tree Map reveals the Impact of the Top 10 Trends in Precision Medicine
The Precision Medicine Tree Map highlights the Top 10 Trends in Precision Medicine in 2025. These include biomarkers, microbiome, ethical AI, data integration, and drug discovery technologies. The market also adopts clinical decision support systems, gene editing, and more. These trends reflect how the domain leverages technologies to improve disease stratification, treatment personalization, and patient care effectiveness.
Global Startup Heat Map covers 1268 Precision Medicine Startups & Scaleups
The Global Startup Heat Map showcases the distribution of 1268 exemplary startups and scaleups analyzed using the StartUs Insights Discovery Platform. It highlights high startup activity in the US and the UK, followed by India and Germany. From these, 20 promising startups are featured below, selected based on factors like founding year, location, and funding.

Want to Explore Precision Medicine Innovations & Trends?
Top 10 Emerging Trends in Precision Medicine [2025 and Beyond]
1. AI Integration
AI algorithms analyze complex medical data to identify diseases at earlier stages and improve patient outcomes. This enables the development of treatment strategies tailored to individual genetic profiles.
Moreover, AI improves clinical trial design by identifying suitable participants and predicting responses. This increases the likelihood of successful outcomes.
Solutions like AI-driven drug discovery platforms actively predict molecular interactions and identify potential therapeutic compounds. For instance, Insitro uses machine learning to analyze vast datasets of chemical and biological markers. This shortens the development cycle of new medicines.
AI-powered CDSS platforms also assist clinicians in making informed decisions by analyzing patient data and providing evidence-based recommendations.
Moreover, AI-enabled genomics tools rapidly analyze whole-genome sequences (WGS) for identifying mutations and developing personalized therapies. Notably, DeepMind’s AlphaFold predicts protein structures with remarkable accuracy. This allows researchers to better understand disease mechanisms.
AI further tailors treatment plans to individual patients by analyzing genetic, molecular, environmental, and lifestyle factors. For example, the integration of AI in personalized medicine enables precise, data-driven treatments tailored to individual patients.
According to Precedence Research, the global AI market size in precision medicine is anticipated to reach around USD 49.49 billion by 2034, growing at a CAGR of 35.80% from 2025 to 2034.
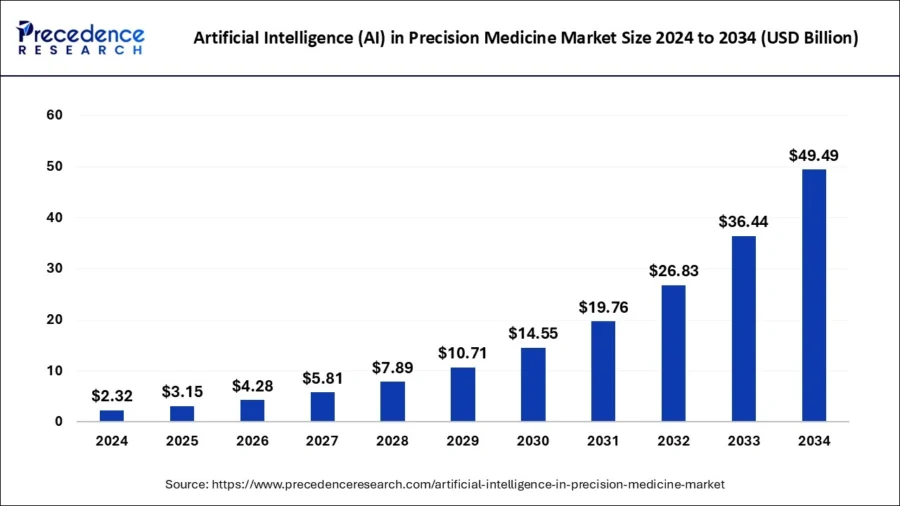
Source: Precedence Research
Multiomic Health delivers Metabolic Syndrome Therapeutics
UK-based startup MultiOmic Health develops AI-enabled precision therapeutics targeting metabolic syndrome-related diseases.
The startup’s MOHSAIC platform integrates multi-omics data – including genomics, proteomics, metabolomics, and epigenomics – with longitudinal clinical information to construct computational systems biology models. Researchers then refine these models through targeted wet lab experiments.
This methodology enables the identification of distinct patient subpopulations, or endotypes, characterized by molecular disease drivers. It also facilitates the discovery of novel drug targets and the development of corresponding patient-stratifying biomarkers.
Moreover, the startup focuses on specific endotypes to streamline clinical trials, shorten their duration, reduce their size, and increase the chances of therapeutic success.
Further, MultiOmic Health converts the treatment landscape for multifactorial conditions such as type 2 diabetes, chronic kidney disease, and non-alcoholic fatty liver disease. It also delivers targeted therapies that address the underlying molecular mechanisms of these diseases.
Clinsight provides AI-powered Prostate Pathology
Swedish startup Clinsight offers AI-powered image analysis algorithms for prostate cancer diagnosis, grading, and prognosis.
The company’s deep learning models analyze digitized prostate biopsy slides, identify cancerous regions, and assign Gleason scores to predict disease progression. Pathologists, urologists, and oncologists use these algorithms within digital pathology workflows to support clinical decision-making.
Moreover, AI-powered image analysis algorithms use whole slide images from diverse clinical sites for training and undergo validation across multiple scanner platforms. They also deliver standardized, automated, and quality-assured evaluations.
2. Precision Oncology
Precision oncology customizes cancer therapy based on a tumor’s molecular profile. This improves outcomes over non-matched treatments. Studies show that patients with non-small cell lung cancer (NSCLC) who receive matched therapies achieve better results.
Precision oncology also brings new treatment options for patients with rare or resistant cancers. In Australia, the MoST program shows that genomic screening identifies actionable genetic biomarkers in 75% of rare cancer patients. This provides access to targeted therapies and improves outcomes.
As a result of these advancements, the global precision oncology market size was estimated at USD 115.8 billion in 2024 and is projected to grow at a CAGR of 8.05% from 2025 to 2030.
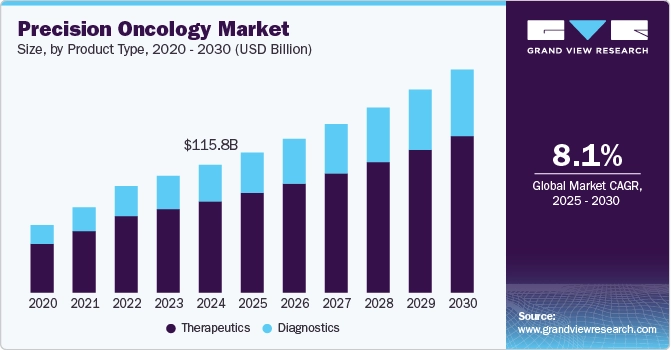
Source: Grand View Research
Next-generation sequencing (NGS) analyzes genetic mutations in tumors to guide personalized treatment strategies. Agendia develops genomic tests like MammaPrint and BluePrint for early-stage breast cancer to support treatment decisions.
Moreover, AI-powered systems deliver context-aware insights using real-world evidence (RWE) and patient-specific factors. For instance, oncology teams adopted AI-enhanced clinical decision support tools to guide treatment decisions.
Guardant Health, Natera, and Exact Sciences develop minimal residual disease (MRD) tests, including Guardant Reveal and Natera Signatera, to enable early intervention and guide personalized treatment strategies.
Additionally, messenger ribonucleic acid (mRNA) vaccines are being tailored to individual tumor profiles. AI-driven tumor organoid modeling is also used to predict treatment responses.
Rosetta Omics specializes in Multi-Omics Precision Oncology
French startup Rosetta Omics develops a multi-omics precision oncology platform that supports personalized cancer treatment decisions. The platform integrates spatial proteomics, metabolomics, and artificial intelligence.
The startup begins its workflow with matrix-assisted laser desorption/ionization (MALDI) mass spectrometry imaging to generate spatially resolved molecular maps of tumor biopsies.
It uses laser capture microdissection to isolate specific tissue regions and applies LC-MS/MS to identify and quantify proteins and metabolites with high resolution. The platform analyzes thousands of analytes from minimal biopsy material and overcomes the limitations of conventional immunohistochemistry.
Machine learning algorithms process the molecular profiles after data acquisition to rank therapeutic options and predict treatment responses. Further, the platform integrates into standard diagnostics for clinicians to choose therapies and support drugmakers in finding biomarkers and new targets.
BioCreatrix builds a Personalized Cancer Vaccine Platform
Hong Kong-based startup BioCreatrix provides a precision medicine platform to advance individualized oncology care. It integrates personalized cancer vaccines, CAR-NK immunotherapies, and liquid biopsy diagnostics.
Additionally, the startup’s vaccine pipeline uses genomic sequencing and machine learning to identify patient-specific tumor neoantigens. It formulates these neoantigens into investigational vaccine pools for clinical administration.
In parallel, BioCreatrix engineers CAR-NK cells with tri-specific tumor-targeting receptors that combine natural cytotoxicity with CAR-mediated mechanisms. This enables dual-mode tumor cell elimination.
Through its Telotech division, the startup provides ultra-deep sequencing of ctDNA and CTCs to enable early cancer detection, therapy matching, and real-time treatment monitoring. This further enables BioCreatrix to deliver tailored immunotherapies and diagnostics for early intervention and better patient outcomes.
3. Personalized Diagnostics
Personalized diagnostics use genetic, proteomic, and metabolic data to detect diseases with greater accuracy and at earlier stages. This enables faster diagnoses and timely treatment.
Customized diagnostics improve health outcomes by enabling proactive disease management and monitoring. They match patients with treatments tailored to their condition and eliminate the trial-and-error approach of traditional methods.
Healthcare providers also analyze a patient’s biological makeup to design treatments that work better and cause fewer side effects. This improves care for chronic diseases and cancer.
The global precision diagnostics market is projected to grow to USD 207 billion by 2033, with a CAGR of 10.09% from 2025 to 2033, according to IMARC Group.
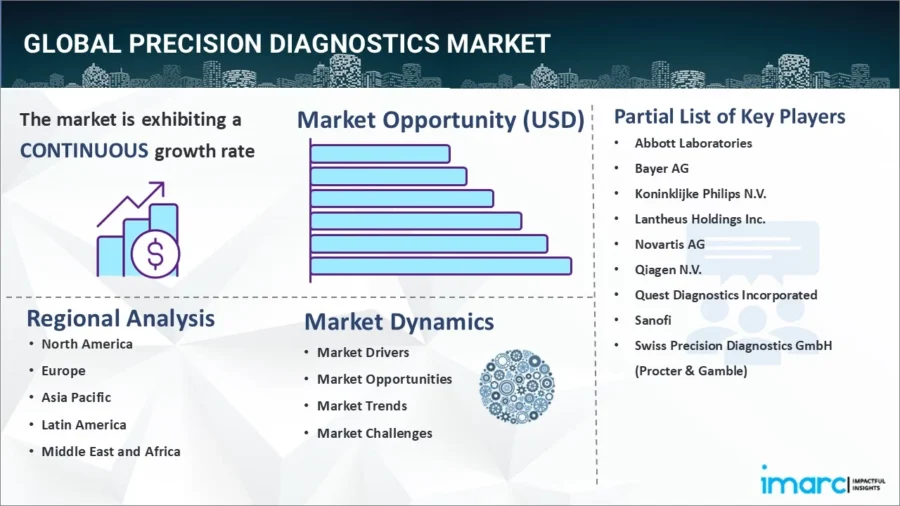
Source: IMARC Group
Next-generation sequencing also enables the detection of disease-related genetic mutations. For example, Tempus AI uses NGS to generate genomic profiles for cancer patients and supports personalized treatment decisions.
Digital polymerase chain reaction (PCR) precisely quantifies nucleic acids and detects low-abundance genetic material. AI-enhanced digital nucleic acid amplification tests (dNAATs) improve sensitivity and specificity in detecting infectious diseases and genetic mutations.
Further, AI and machine learning (ML) algorithms process vast datasets to identify patterns and predict disease risks. Quibim‘s AI-powered tools, such as QP-Prostate and QP-Brain, assist in diagnosing prostate and brain conditions by analyzing medical imaging data.
DATABIOMIX provides a Microbiomics Platform
Swiss startup DATABIOMIX offers an integrated microbiome bioinformatics platform. It delivers high-resolution analysis for clinical diagnostics, microbial genomics, and therapeutic development.
The startup’s microbiomics platform processes 16S rRNA metagenomic data to identify species-level microbial biomarkers. This supports disease association studies and dietary intervention assessments.
Additionally, its microbial genomics platform assembles and annotates complete bacterial genomes from single long-read sequencing runs. This enables genomic surveillance, functional screening, and regulatory submissions.
This way, DATABIOMIX automates complex bioinformatics workflows for personalized diagnostics and precision microbiome research.
Exsegen improves Brain Tumor Diagnostics
Indian startup Exsegen builds a precision diagnostics platform for brain tumors. It combines liquid biopsy, AI-powered pathology, and clinical trial management through its proprietary technologies, GalenPath and Elsy.
Additionally, Elsy streamlines clinical trial workflows by managing patient data, genomic sequences, biosample tracking, and regulatory compliance. GalenPath combines histopathology and molecular data to generate integrated diagnostic reports.
Moreover, the startup’s liquid biopsy solution analyzes circulating tumor DNA from blood samples using machine learning to identify tumor-specific molecular signatures. Further, its tissue-based NGS test performs DNA methylation profiling to classify brain tumor subtypes.
4. Drug Discovery Technologies
Emerging drug discovery technologies address high costs, long timelines, and low success rates associated with conventional personalized drugs. These technologies, including generative AI models, molecular docking, and more, improve the design and validation of novel therapeutics. They also optimize candidate selection to align with specific disease profiles.
Generative AI models for de novo drug discovery create new molecular structures by expanding chemical space and improving target specificity. Moreover, the integration with molecular docking refines these outputs by simulating binding affinity to target proteins.
Additionally, genomics and next-generation sequencing improve the rapid and affordable sequencing of genomes. They identify genetic variants linked to disease susceptibility, drug response, and prognosis, which form the backbone of personalized medicine.
Epigenomic tools further analyze DNA and histone modifications that regulate gene expression without changing the genetic code. These tools and molecular phenotyping techniques construct detailed molecular maps of disease mechanisms.
Moreover, imaging technologies, including radiomics, extract quantitative features from medical images such as CT, MRI, and PET scans to support diagnosis, prognosis, and treatment planning. These technologies offer objective, data-driven insights, especially in oncology, where they characterize tumors and assess how they respond to therapy.
These solutions reduce discovery time, lower attrition, and increase the delivery of precision therapies. They also embed AI into drug development to redesign and deploy personalized treatments.
PolygenRX offers a Drug Discovery Platform
Australian startup PolygenRx develops a precision medicine platform that integrates genetic data to inform drug discovery and treatment matching for complex disorders.
The startup analyzes genome-wide single-nucleotide polymorphism (SNP) data from patients. It combines this data with genome-wide association study (GWAS) findings to assess genetic risk within drug-responsive biological pathways.
This process generates a pharmagenic enrichment score (PES), quantifying an individual’s genetic burden in pathways with known pharmacological targets. Moreover, PolygenRx’s platform evaluates multiple genetic factors related to drug targets, which improve clinical interpretability.
The PES is scaled against a reference population to identify individuals most likely to benefit from specific treatments. This allows for patient stratification for clinical trials and informs therapeutic decisions.
The platform uses fixed germline genetic variations to provide a stable approach for diseases like cancer, neurodegenerative, autoimmune, and psychiatric disorders. PolygenRx further optimizes treatment outcomes by aligning patients with therapies tailored to their genetic profiles.
Rayca Precision advances Multimodal Drug Discovery
UK-based startup Rayca Precision develops RSA2 and OncoCrest, two AI-driven platforms that improve multimodal drug discovery and precision oncology.
RSA2 automates bioinformatics workflows by analyzing transcriptomic and genomic sequencing data. It also predicts 3D protein structures and maps drug-target interactions, thereby streamlining therapeutic candidate design.
OncoCrest applies deep learning to patient-derived molecular profiles to predict treatment responses, identify fusion events, and support survival analysis. This improves personalized cancer care.
Additionally, the startup integrates these platforms into a closed-loop system that continuously refines therapeutic designs based on laboratory and preclinical feedback. Rayca Precision combines computational modeling and experimental validation to develop therapies for hard-to-treat targets.
5. Biomarkers
Biomarkers allow clinicians to classify patients based on genetic, molecular, or cellular characteristics. This ensures that the treatments are tailored to individual profiles.
Moreover, incorporating biomarkers into diagnostics leads to earlier and more precise disease detection. This is particularly beneficial in conditions like cancer and neurodegenerative diseases, where early intervention is crucial.
As their applications expand across disease areas and research pipelines, the demand for biomarker technologies continues to surge. The global biomarkers market size is estimated to grow up to USD 198.58 billion by 2035, at a CAGR of 11.1% during the forecast period till 2035.
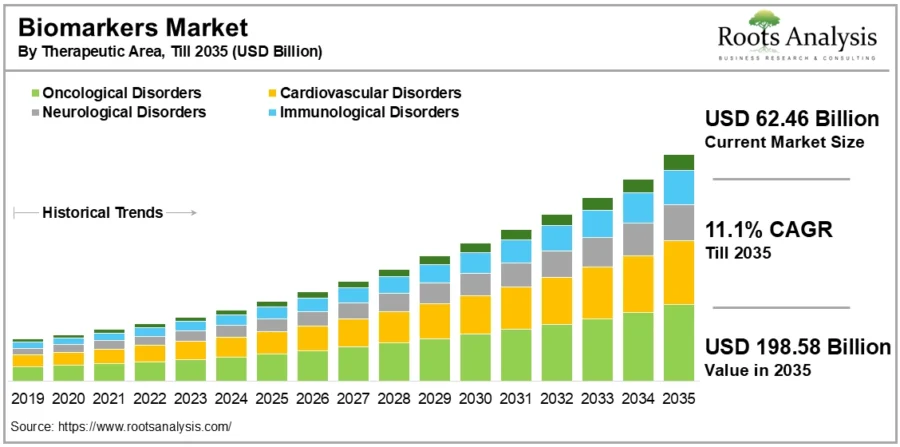
Source: Roots Analysis
Multi-omics platforms provide a detailed view of disease mechanisms, which improves biomarker discovery and patient stratification. For example, in 2024, the omics technologies captured an estimated 53% share in the biomarker market due to their ability to conduct a detailed molecular data analysis.
Additionally, AI analyzes complex datasets to identify novel biomarkers and predict treatment responses. The AI tool FaceAge, for example, estimates biological age from facial features to predict cancer survival outcomes.
OncoDecipher provides a Biomarker-driven Therapy Platform
Israeli startup OncoDecipher develops an AI-powered platform that identifies and analyzes novel genetic variations, termed OnD biomarkers. The platform uncovers hidden cancer biomarkers and expands personalized treatment options.
Additionally, the platform processes clinical and genetic patient data using computational and bioinformatic tools. It detects DNA sequence variations, predicts their biological effects, and assesses therapeutic outcomes.
Moreover, OncoDecipher’s approach reveals previously undetected biomarkers, thereby broadening the scope of precision oncology. The startup further uses molecular techniques to validate biomarkers and improve the precision of cancer diagnostics and therapeutics.
Quant Biomarkers provides AI-powered Biomarker Discovery
Swiss startup Quant Biomarkers builds an AI-driven biomarker platform that enables early detection and personalized intervention strategies for aging-related chronic diseases. The startup further supports healthy longevity and reduces the burden of chronic illness.
The startup’s technology integrates standard-of-care blood biomarkers with proprietary machine learning algorithms to predict disease progression. It initially focuses on conditions like chronic kidney disease through its RenoPredict clinical decision support system.
Additionally, the platform uses tissue-specific biomarker panels and digital twin applications to provide real-time health insights and personalized medical protocols. This improves preventive care by identifying therapeutic targets and optimizing intervention timing.

6. Cell and Gene Therapies
CGTs leverage a patient’s cells or genetic material to reduce immune rejection risks for improving treatment efficacy and advancing precision medicine.
For example, chimeric antigen receptor (CAR-T) cell therapies show strong efficacy in cancer treatment. Lifileucel (Amtagvi) treats advanced melanoma, while Obecabtagene autoleucel (Aucatzyl) targets relapsed B-cell acute lymphoblastic leukemia.
The global CGT market size is predicted to increase from USD 25.03 billion in 2025 to approximately USD 117.46 billion by 2034, expanding at a CAGR of 18.7% from 2025 to 2034.
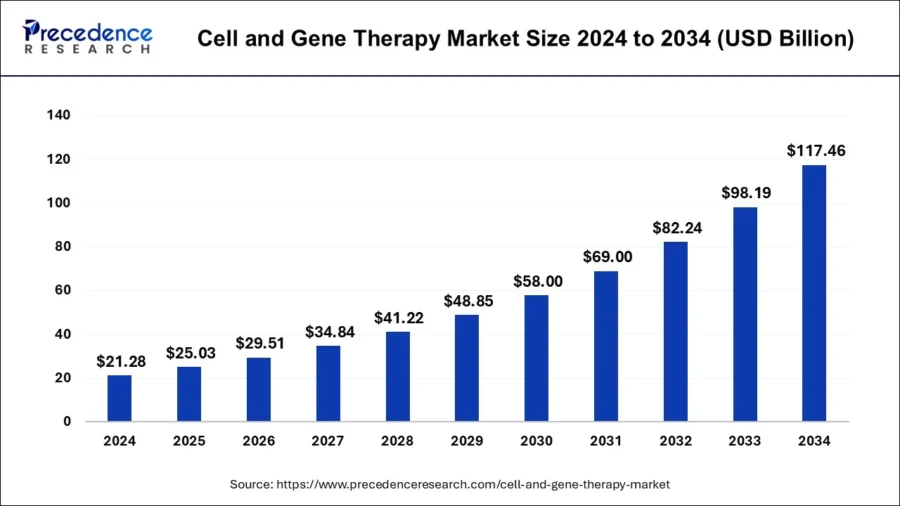
Source: Precedence Research
Further, solutions like adeno-associated virus (AAV) vectors deliver therapeutic genes to target cells. A noteworthy example is that of atidarsagene autotemcel (Libmeldy), which treats metachromatic leukodystrophy by delivering functional copies of the ARSA gene.
Base and prime editing allow for precise modifications without causing double-strand breaks. Additionally, decentralized manufacturing and process automation systems produce CGTs closer to patients and improve accessibility.
MFX (MicrofluidX) develops an Automated Bioprocessing Platform
UK-based startup MFX (MicrofluidX) builds Cyto Engine, an automated bioprocessing platform that speeds up the development and manufacturing of cell and gene therapies.
The platform integrates microfluidic bioreactor cassettes with real-time process analytics and machine learning algorithms. This enables precise control over cell culture conditions across research and production scales.
Moreover, the platform’s modular design supports scale-up and scale-out, while its manufacturing stack allows multiple bioreactors to operate within a single compact footprint.
MFX combines process automation with data visualization to improve reproducibility and efficiency in cell therapy workflows. Further, the startup streamlines the transition from laboratory research to clinical manufacturing while reducing costs.
Talos Smart Technology offers AI and Microfluidic Solutions
Spanish startup Talos Smart Technology develops an integrated platform for decentralized CAR-T cell therapy manufacturing. It combines organ-on-chip and lab-on-chip systems with AI, image recognition, multi-omics analysis, and robotics to automate T-cell culture and monitor therapeutic efficacy.
Additionally, the platform employs microfluidic bioreactors and reinforcement learning algorithms to adjust flow parameters. This improves cell growth and reduces contamination risks.
Further, the startup localizes production within hospitals to minimize logistical delays, lower costs, and enable rapid, patient-specific therapy adjustments. This approach streamlines CAR-T therapy delivery and makes it more accessible and tailored to individual patient needs.
7. Multi-Omics
Multi-omics solutions combine genomics, transcriptomics, proteomics, metabolomics, and epigenomics to enable a deeper understanding of complex disease mechanisms and identify novel therapeutic targets.
Multi-omics also facilitates the development of tailored therapies by considering individual genetic and molecular profiles. This increases treatment efficacy and reduces adverse effects.
Moreover, researchers identify more accurate biomarkers for disease diagnosis, prognosis, and treatment response by analyzing multiple omic layers. Genomics enables whole-genome sequencing to identify genetic variants associated with diseases.
Additionally, proteomics performs the large-scale study of proteins to provide insights into disease mechanisms and potential biomarkers. The University of Chicago’s Proteomics Platform uses advanced mass spectrometry to analyze protein expression and support disease diagnosis and treatment monitoring.
At the same time, metabolomics analyzes metabolites in biological samples to capture the physiological state and track disease progression. Creative Proteomics offers targeted metabolomics services that identify disease biomarkers and improve diagnostic accuracy.
Further, spatial omics maps molecular data within the spatial context of tissues to understand disease microenvironments.
As per Roots Analysis, the multiomics market size is projected to grow from USD 3.10 billion in 2025 to USD 12.65 billion by 2035, representing a CAGR of 15.09% during the forecast period till 2035.
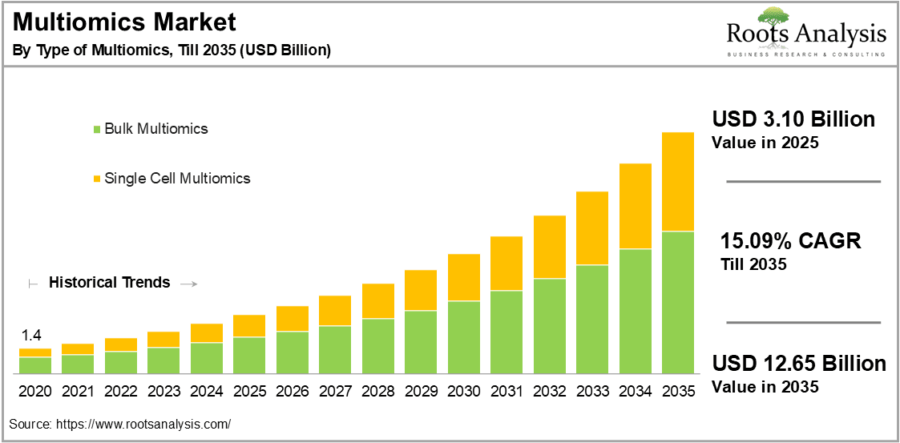
Source: Roots Analysis
CODETTA BIO facilitates Microbead-based Multiplexed Target Capture and Analysis
US-based startup Codetta Bio develops Concerto System, a platform that quantifies proteins, DNA, and RNA from a single sample. It utilizes microbead-based target capture with digital PCR, real-time PCR, and immunoPCR to enable high-sensitivity, high-throughput analysis.
It also eliminates non-specific signals through a noise-canceling assay design to ensure precise measurements in complex, multiplex environments. The platform also combines digital and analog detection to expand the dynamic range and support accurate quantification.
This unified workflow reduces sample usage, increases efficiency, and improves oncology, immunology, and precision medicine research.
GenomeArc offers a Genomic Diagnosis and Discovery Platform
Canadian startup GenomeArc provides Horizon, a multi-omics clinical genomic platform for rapid diagnosis and discovery in rare diseases and cancer.
The platform processes whole-genome and exome variant files and integrates genomic, transcriptomic, and proteomic data with AI-driven analysis to classify variants according to ACMG guidelines.
It further incorporates single-cell transcriptomics and proteomics to provide tissue and cell-type-specific insights into gene expression. The platform supports pharmacogenomic profiling by linking gene variants to drug response data.
8. Microbiome
Each person’s microbiome composition shapes their risk of developing diseases such as inflammatory bowel disease (IBD), obesity, and cancer. Therefore, clinicians analyze microbial profiles to predict disease risks more accurately and design personalized preventive strategies.
The microbiome also alters how individuals metabolize medications, influencing drug efficacy and the risk of adverse reactions. Understanding these interactions enables clinicians to select therapeutics and determine dosages more precisely.
For example, studies show that the gut microbiome directly shapes immune responses, affects vaccine effectiveness, and alters disease susceptibility. Also, the therapeutic potential of microbiome modulation has been a focal point.
Scientists engineer probiotics to produce specific metabolites, like short-chain fatty acids (SCFAs), to improve gut health and treat metabolic disorders. This marks a shift toward microbiome-based therapeutics for a range of health conditions. The global microbiome market size is poised to reach USD 3.95 billion by 2032, growing at a CAGR of 17.7% during the forecast period (2025-2032).
Perseus Biomics enables Microbiome Profiling
Belgian startup Perseus Biomics provides DynaMAP, a microbiome profiling technology that delivers strain-level resolution using high-density optical mapping.
The technology employs fluorocoding, molecular combing, and single-molecule imaging to generate taxonomic barcodes from long DNA fragments. This enables precise identification of microbial strains without amplification or sequencing.
Additionally, its benchtop device, MetaMAP, integrates this technology into laboratory workflows to facilitate rapid and scalable microbiome analysis. Perseus Biomics streamlines the profiling process to support personalized nutrition, biotic development, epidemiology, and precision medicine.
Melius MicroBiomics offers Genetically Engineered Microbes for Inflammation Therapy
Canadian startup Melius MicroBiomics engineers genetically engineered microbial medicines (GEMM) to improve gut health. It equips beneficial bacteria with genetic traits to improve resilience and colonization in inflamed intestinal environments.
The company’s BioPersist platform enables engineered microbes to utilize alternative energy sources produced during inflammation, allowing them to maintain metabolic function and exert anti-inflammatory effects.
Additionally, the BioColonize platform introduces genes encoding binding proteins that facilitate adherence to intestinal epithelial cells. This promotes stable colonization and mucosal healing. Further, the platforms collectively address the limitations of traditional probiotics.
9. Clinical Decision Support Systems
CDSS tools drive precision medicine by analyzing patient data, including genetic, molecular, and clinical information, to deliver evidence-based, personalized recommendations. These tools reduce diagnostic errors and optimize therapy selection based on individual risk profiles.
For example, Mayo Clinic analyzes low-dose CT scans to detect lung cancer by identifying potential tumors and reducing unnecessary biopsies and surgeries.
The global clinical decision support systems market size is estimated to reach around USD 8.10 billion by 2034, expanding at a CAGR of 8.30% from 2024 to 2034.
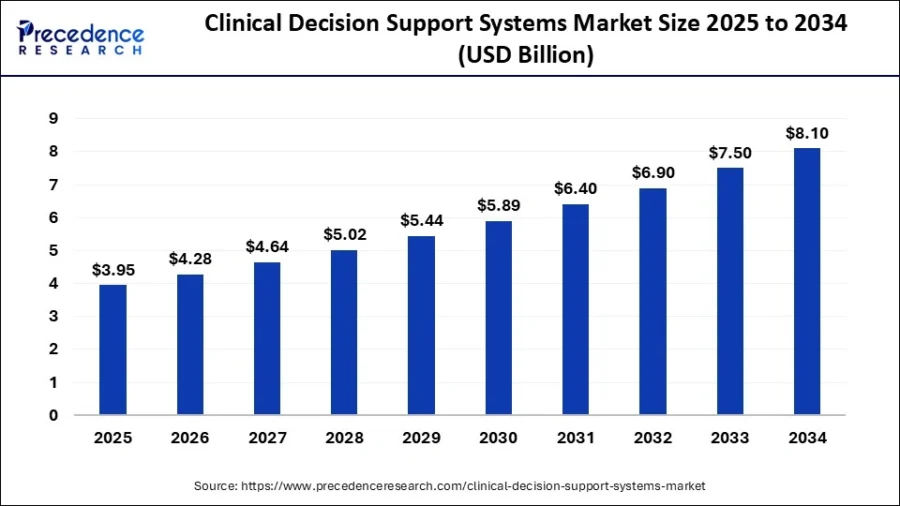
Source: Precedence Research
Further, Ava Industries integrates Ava Scribe with electronic medical records to transcribe clinical notes in real time, reduce documentation time, and improve workflow efficiency. This way, clinicians use up-to-date patient information to make timely, individualized treatment decisions based on medical histories and evolving clinical data.
Predictive analytics in CDSS identifies high-risk patients using genomic markers, lifestyle factors, and historical data. This enables early interventions and personalized treatment pathways. Integrated with wearable devices, CDSS continuously monitors vitals and biomarkers to trigger alerts for deviations requiring targeted interventions.
Vita Innovations offers a Generative-AI-based Platform
US-based startup Vita Innovations develops EmerGenAI, an AI-powered platform for emergency departments. It streamlines workflows by automating patient intake and improving clinical decision-making.
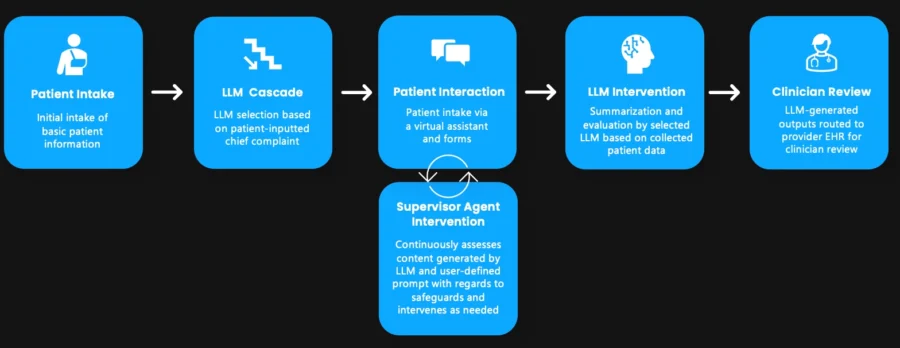
The platform employs a multi-agent system comprising patient care AI, which gathers patient information through virtual interactions and electronic health record (EHR) retrieval.
Additionally, clinical decision support AI generates patient summaries, emergency severity index (ESI) scores, protocol guidance, and supervisor AI, which ensures content moderation and bias control.
Further, the platform collects data on social determinants, extracts medication details using image recognition, and matches patients to local social services based on their needs.
OneDrug designs Smart Point-of-Care Devices
Canadian startup OneDrug develops a smart point-of-care pharmacogenetic testing device for real-time analysis of patients’ genetic profiles. It identifies variations in drug-processing proteins to inform treatment decisions.
The device operates without specialized expertise and delivers results within 20 minutes through a user-friendly interface compatible with laptops or smartphones. It securely stores the data in the cloud.
Additionally, the startup’s smart health app complements the device to provide a unified platform for telemedicine, medication intelligence, and precision patient management. It also features advanced interaction analytics and multilingual support.
10. Ethical AI
Precision medicine relies heavily on sensitive data, and ethical AI frameworks prioritize data governance to protect patient information and ensure fair use as AI adoption accelerates in the sector.
Moreover, AI models trained on non-representative datasets perpetuate health disparities by generating inaccurate personalized treatments and predictions. Ethical AI in precision medicine uses inclusive and diverse genomic, clinical, and environmental data to deliver equitable therapeutic outcomes precisely tailored to diverse patient populations.
Solutions like explainable AI (XAI) make AI decisions interpretable and enable clinicians to understand and trust AI-driven recommendations. For instance, IIT Indore developed a quantum AI nanotechnology integrating explainable AI to detect genetic mutations for early cancer detection.
SilicoGene offers a No-Code AI Development Platform
Dutch startup SilicoGene builds EkaFlow, a no-code AI platform for drug discovery. It allows health-tech teams to process omics data, build machine learning models, and deploy them at scale.
The platform operates through a drag-and-drop interface. It allows healthcare professionals to build bioinformatics pipelines for transcriptomics, genomics, and proteomics data without programming expertise.
Additionally, the platform integrates tools for data extraction, transformation, and loading (ETL), model versioning, and automated scaling. It also provides access to on-demand GPU resources to support computationally intensive tasks. Further, EkaFlow offers HIPAA-compliant infrastructure options to ensure data security and privacy.
Leeaf provides an XAI-driven Fertility Treatment Platform
Czech Republic startup Leeaf offers a digital reproductive health platform that integrates personalized patient support with clinical decision tools to enhance fertility care.
The startup also provides a companion mobile application that enables women to track menstrual cycles, manage fertility treatments, and monitor pregnancy progression. Moreover, it secures the storage and sharing of medical data.
Concurrently, the Leeaf physician portal aggregates patient lifestyle, medical history, and risk factors to assist clinicians in tailoring reproductive treatments. Further, Leeaf enables real-time data exchange between patients and healthcare providers to streamline communication and support individualized care plans.
Discover all Precision Medicine Trends, Technologies & Startups
Emerging trends include spatial and temporal omics technologies, quantum machine learning, and digital patient twins. Advances in metabolomics and in-silico clinical trials further improve targeted intervention design. These technologies reshape disease prevention, risk assessments, and therapeutic strategies, making care delivery more proactive and precise.
The Precision Medicine Trends & Startups outlined in this report only scratch the surface of trends that we identified during our data-driven innovation & startup scouting process. Identifying new opportunities & emerging technologies to implement into your business goes a long way in gaining a competitive advantage.
![Discover the Top 10 Trends in Precision Medicine [2025]](https://www.startus-insights.com/wp-content/uploads/2025/05/Precision-Medicine-Trend-SharedImg-StartUs-Insights-noresize-420x236.webp)


![Discover the Top 10 AI Trends in Healthcare [2025]](https://www.startus-insights.com/wp-content/uploads/2025/04/AI-Trends-in-Healthcare-SharedImg-StartUs-Insights-noresize-420x236.webp)
![AI in Healthcare: A Strategic Guide for Industry Leaders [2025-2030]](https://www.startus-insights.com/wp-content/uploads/2025/03/AI-in-Healthcare-SharedImg-StartUs-Insights-noresize-420x236.webp)





