Accelerate Productivity in 2025
Reignite Growth Despite the Global Slowdown
Executive Summary: What are the Top 10 Trends Shaping the Future of Pharma (2026-2030)?
Pharma is undergoing a high-stakes transformation as aging populations, patent cliffs, and rising R&D costs drive a shift toward smarter, faster, and more resilient drug pipelines.
- Next-Generation Advanced Therapies (NGAT): Scalable gene editing, base editing, and allogeneic cell therapies are expanding into common diseases. Cell and gene therapy (CGT) startups secured USD 26 billion in funding.
- Generative & Agentic AI: Generative AI now functions as a full-stack molecular engine. Global investment reached USD 45 billion, with AI-designed drugs progressing to Phase II clinical trials.
- Quantum Computing for Drug Discovery: Quantum platforms are resolving molecular design bottlenecks within minutes. Pharma-focused investment exceeded USD 1.25 billion in Q1 2025, with companies reducing hit-to-lead timelines by up to 20 times.
- 3D-Printed Oral Medicines: US Food and Drug Administration (FDA)-approved capsules and personalized polypills demonstrate additive manufacturing’s readiness for scale. The market is projected to reach USD 789 million by 2034.
- Robotic Aseptic Fill-Finish: Fully enclosed ISO-5 robotic systems lower contamination risks and cut validation costs. Since 2024, over EUR 1.1 billion has flowed into next-gen filling lines, advanced robotics, and energy-efficient cleanrooms.
- Integrated Digital Twins: Pharma is linking R&D, manufacturing, and clinical trials through synthetic data and digital twins. Companies like AstraZeneca and Merck report 56% productivity gains and 67% faster development using AI-powered twins.
- Smart Design & Continuous Manufacturing: Recent investments indicate a shift from batch to continuous production. Systems like Lilly’s Medicine Manufacturing Foundry and Johnson & Johnson’s Flex Line reduce waste and accelerate product launches.
- Blockchain Traceability: Blockchain is now critical for cold-chain logistics and anti-counterfeit safeguards. Pilots by Merck’s M-Trust, TrueCold, and DHL show improved compliance and reduced product loss.
- Net-Zero & Low-Carbon Operations: With 53% of pharmaceutical firms committed to the United Nations’ Race to Zero initiative, capital expenditure is increasingly directed toward biomethane, renewable energy, and low-emission facilities.
- Agile & Convergent Regulation: Real-time regulatory reviews and platform-level FDA designations, such as Real-Time Oncology Review (RTOR) and Advanced Manufacturing Technology (AMT), are accelerating drug approvals by 6 to 12 months.
Macro Drivers Reshaping the Future of the Pharma Industry
Demographic & Epidemiology
By 2030, 1 in 6 people worldwide will be 60 or older. This expands long-term needs for chronic care, neurology, and specialty treatments.
Conditions like Alzheimer’s and diabetes are growing sharply. In 2025, 7.2 million Americans over 65 will be living with Alzheimer’s. Diabetes now affects 11.1% of the global adult population, with prevalence projected to grow by 46% by 2050.
Further, non-communicable diseases (NCDs) now account for 75% of global deaths, with 18 million occurring before age 70. This raises the strategic need for earlier intervention pipelines.
Cost & Pricing Pressure
Global inflation in medical spend is forecast to hold at 10.4% in 2025, nearly matching the 2023 rate, which was the highest in the past decade.
In the US, employers face 5.8% cost growth on average, with premiums hitting USD 25 572 per family in 2024.
The US Centers for Medicare & Medicaid Services (CMS) has set maximum fair prices for the first 10 high-expenditure drugs, which could reduce net spending by 22%.
Globally, 118 biologics will lose patent protection by 2034, opening up a USD 232 billion opportunity for biosimilars.
Capital & Funding
In 2024, VC funding in biotech increased year-over-year, reaching USD 21.4 billion. However, Q1 2025 showed signs of a plateau, with deal volume for Biopharma dropping by 20.2% year-on-year.
Despite this, capital remains concentrated, as median private biotech rounds held strong at USD 93 million. This reflects investor preference for platform companies with scalable, high-impact potential.
At the same time, the pharma industry faces a looming USD 236 billion patent cliff by 2030. Keytruda alone is losing its USD 29.5 billion exclusivity in 2028. As a result, licensing activity is heating up, with seven deals in 2024 topping USD 1 billion each.
10 Futuristic Pharmaceutical Trends to Look for During 2025-2035
1. Next-Generation Advanced Therapies: A Multi-Billion Dollar Market
Next-generation advanced therapies are gaining real traction as gene editing, base editing, personalized mRNA vaccines, and allogeneic cell therapies are entering mainstream development.
For example, ElevateBio raised USD 525 million to scale its BaseCamp CGT manufacturing and modular lentiviral vector platform. This is a key infrastructure as pharma shifts from ultra-rare to chronic, high-volume indications.
Simultaneously, seed and Series A rounds for cell and gene therapy (CGT) startups also tripled to USD 484 million in Q3 2024. In total, USD 26 billion has flowed into therapeutics venture funding this year alone. Meanwhile, a wave of 10 biotech IPOs in early 2025 signals a reopened public market for late-stage players.
On the pipeline front, developers are now going after high-prevalence indications. 79 new gene therapy trials launched in Q1 2025, with 57% in oncology. This signals a shift from rare disease to large-population targets.
Caribou Biosciences’ CB-010 showed early efficacy in an off-the-shelf CRISPR CAR-T trial. This marked a step toward mass-manufacturable allogeneic cell therapies. In cardiology, Verve Therapeutics secured FDA fast-track status for its base editing candidates targeting PCSK9 and LPA, with first human data expected in Q2 2025.
On the manufacturing front, industrial-scale platforms like Catalent’s UpTempo AAV system cut CMC timelines, while Novartis’ gene therapies are piloting continuous vector production to drive cost efficiency.
These advances are tackling the toughest bottlenecks in cell and gene therapy manufacturing and laying the groundwork for NGATs to move from the lab to broader, commercial use.
Business Impact & Opportunities
Curative Therapies Justify Premium Pricing Models
Long-term data from Casgevy show that 93% of sickle-cell patients remained free of pain crises for at least 12 months after a single CRISPR treatment. This strengthens the economic case for one-time, high-cost therapies and enables annuity or value-based pricing models to replace chronic-care reimbursement.
Multi-Decade Growth Runway
Global revenues from cell and gene therapies are projected to grow to USD 117 billion by 2034. This market trajectory supports pipeline realignment and late-stage asset acquisitions. It also drives M&A-based portfolio diversification as traditional drug portfolios face USD 180 billion in looming patent expiries.
Accelerated Regulatory Pathways Boost Speed-to-Market
A wave of regulatory accelerators is shortening commercialization timelines. Cellares’ automated manufacturing platform received the FDA’s advanced manufacturing technology (AMT) designation; Verve Therapeutics earned Fast-Track status for its base-editing PCSK9 candidate. These tags accelerate approval timelines.
Payers Adopt Outcome-Based Models for Million-Dollar Therapies
Germany’s national health system launched Hemgenix, a gene therapy for hemophilia, under a success-based reimbursement model in June 2025. This demonstrates growing payer openness to alternative payment structures tailored for high-cost, one-time therapies.
Real-World Deployments & Pilots
NHS Creates a Scalable Model for Personalized Vaccine Trials
The UK’s NHS Cancer Vaccine Launch Pad streamlines trial enrollment for individualized mRNA cancer vaccines. It’s the first model aimed at matching thousands of patients to precision immunotherapies at scale.
Thermo Fisher’s USD 2 billion Commitment for Vector Industrialization
In March 2025, Thermo Fisher committed USD 2 billion to expand US viral vector and CGT capabilities. This signals that large-scale vector production is becoming the critical infrastructure for advanced therapies.
Spotlighting an innovator: CellVoyant
CellVoyant is a UK-based startup that uses AI-guided live-cell imaging to optimize stem cell differentiation. It captures high-resolution images of cells in culture and analyzes them in real time using proprietary models, FateView, and FateCast.
These models extract spatial and temporal data from millions of cells to predict cell fate and dynamically adjust protocols. The system enables early monitoring, precise predictions, and higher yields of target cells.
By reducing batch failures and shortening timelines, it reduces production costs. The platform enhances scalability, reliability, and efficiency in cell manufacturing.
CellVoyant turns manual stem cell processes into a data-driven platform capable of producing any cell or tissue type at scale. The startup raised GBP 7.6 million in its first round.
2. Generative & Agentic AI: Reduced R&D Timelines
AI agents and generative AI solutions form molecular operating systems that design, simulate, test, and optimize drugs in autonomous cycles.
Global venture capital investment in GenAI reached USD 45 billion in 2024, nearly doubling from USD 24 billion in 2023. Funding for generative AI applications in pharma is also surging. For instance, Pathos AI raised USD 365 million in Series D to advance oncology drug development.
Tech giants like Recursion developed BioHive-2, the largest NVIDIA-powered pharma supercomputer. Also, NVIDIA’s BioNeMo cloud provides chemistry APIs integrated with large language models (LLMs) for drug design.
Clinically, AI-generated drugs are moving deeper into the pipeline. Insilico Medicine’s INS018_055 entered Phase II clinical trials, which is the first GenAI-designed candidate to reach mid-stage trials. Isomorphic Labs is also initiating clinical trials for its AI-developed oncology drugs by late 2025.
These efforts are supported by advances like Google’s AlphaFold 3, which nearly doubles prediction accuracy for protein-ligand interactions and accelerates hit discovery. Pfizer’s GenAI copilots, deployed via AWS, also saved 16 000 researcher-hours annually while cutting infrastructure costs by 55%.
Manufacturing and regulatory ecosystems are also catching up to these advancements. The FDA’s 2025 draft guidance establishes a credibility framework for AI-generated evidence in drug submissions.
Business Value Drivers of AI-Designed Small Molecule Drugs
Cycle-Time Collapse reduces Development Time from Years to Months
AI automates target identification and compound screening to accelerate early drug discovery. Pfizer’s GenAI copilots, for instance, saved over 16 000 hours of manual work annually.
Precision Improves Hit Quality and Reduces R&D Waste
Tools like AlphaFold 3 enhance structural prediction accuracy and minimize false positives in virtual screening. This leads to better hit quality, fewer synthesis missteps, and lower chances of late-stage trial failure.
Cloud Infrastructure Enables Scalable Compute Access
AI design platforms remove the need for costly on-premise infrastructure and make advanced capabilities accessible to smaller and mid-sized pharma firms.
Real-World Deployments & Pilots
Pfizer’s GenAI Stack Cuts Cost and Boosts R&D Productivity
In a 2025 AWS case study, Pfizer’s deployment of GenAI copilots reduced search time by 80% and saved over 16 000 researcher hours annually. This demonstrates the operational value of AI-augmented drug development.
NVIDIA’s BioNeMo Speeds Up Molecule Design with Generative APIs
NVIDIA’s BioNeMo cloud platform offers pre-built generative models for chemistry tasks. Early adopters report faster hit-to-lead cycles and improved molecular binding.
Recursion’s BioHive-2 Sets a New Standard for AI Drug Design Compute
Recursion’s BioHive-2 delivers 2 exaflops (2 quintillion operations per second) via 504 NVIDIA H100 GPUs. This set a new benchmark for AI-driven drug discovery and enabled large-scale phenotypic screening
Spotlighting an innovator: InVirtuoLabs
InVirtuoLabs is a Swiss startup that builds an AI-powered virtual lab for de novo molecular design. The platform screens molecules using three core modules: InVirtuoMOL for machine learning, InVirtuoGEN for generative chemistry, and InVirtuoSIM for physics-based simulations.
The platform applies multimodal learning, active learning, and generative AI to explore vast chemical space.
InVirtuoLabs raised EUR 2.85 million in seed funding to scale its discovery engine and co-develop therapies for neurological, metabolic, and oncology disorders.
3. Quantum Computing: Reduced Discovery Costs and Late-Stage Failures
Aggressive hardware roadmaps, multi-hundred-million-dollar financings, and regulatory alignment are accelerating quantum computing adoption in the pharma industry.
For example, Pasqal’s roadmap to 1000+ qubits and fault-tolerance sets a five-year target for chemistry-relevant error correction. Also, IBM’s 433-qubit Osprey launch expands the cloud-based quantum capacity. These types of advances support life-science researchers in drug discovery and molecular modeling.
On the software side, quantum-accelerated molecular design is gaining real-world traction. Recursion and NVIDIA launched BioHive-2. It is a petascale supercluster that fuses classical AI with near-term quantum kernels to streamline hit-to-lead workflows.
At the same time, Google opened its Sycamore v2 hardware to pharma users via cloud queues and removed the need for on-prem infrastructure. This enables high-fidelity gate-based simulations while democratizing access.
On the regulatory front, the FDA’s January 2025 draft guidance provides a credibility framework for quantum-generated evidence in IND/NDA filings. This enables sponsors to integrate simulations into clinical submissions.
Further, the funding signals are equally strong. Global quantum technology industry funding surged to USD 1.25 billion in Q1 2025 (125% increase) to support faster molecular simulations, precision drug design, and AI-accelerated quantum workflows.
For example, Diraq’s USD 15 million funding round in 2025 reflects rising investor confidence in scalable quantum platforms, with potential pharmaceutical applications in drug discovery.
Business Impact & Opportunities
Faster R&D Timelines, Faster Market Access
Quantum-powered simulations reduce early discovery timelines. By accelerating hit-to-lead cycles and preclinical modeling, pharma companies bring therapies to market faster.
Lower Discovery Costs, Broader Innovation Access
Quantum acceleration reduces the compute load for complex tasks and optimizes infrastructure cost. Lowered infrastructure costs make high-fidelity molecular simulations accessible to big pharma, biotechs, and support scaling R&D efficiently.
New IP Opportunities Through Custom Algorithms
Co-developed quantum models allow pharma firms to generate proprietary simulation tools and molecular insights. They are high-value digital assets difficult for competitors to replicate. Therefore, the industry creates new forms of intellectual property tied to quantum-informed discovery.
Better Candidate Selection with Fewer Trial Failures
Quantum computing offers more accurate predictions of molecular binding, reaction outcomes, and toxicity profiles. That leads to higher confidence in selecting viable drug candidates and less capital lost on late-stage trial failures.
Real-World Deployments & Pilots
AstraZeneca and IonQ Workflow Achieves 20x Speed-Up in Quantum Chemistry Pipeline
AstraZeneca partnered with IonQ, AWS, and NVIDIA to run a full Suzuki-Miyaura catalytic route using IonQ’s Forte quantum processor and NVIDIA CUDA-Q GPUs inside a pharma-grade VPC. The hybrid workflow delivered 20 times faster time-to-solution and reduced GPU compute costs.
Boehringer – Google Quantum AI Target Cytochrome P450 for Drug Discovery Simulations
Boehringer Ingelheim’s partnership with Google Quantum AI focuses on simulating complex enzymes like cytochrome P450. This improves molecular interaction modeling. The collaboration also enhances accuracy in early-stage drug candidate evaluation and signals quantum computing’s rising role in accelerating pharmaceutical R&D.
Cleveland Clinic and IBM Apply Quantum Methods to Protein Structure Prediction
Cleveland Clinic and IBM researchers used quantum computing techniques to explore new approaches to protein structure prediction. The study demonstrated how quantum algorithms complement classical tools in resolving complex molecular folding problems.
Spotlighting an innovator: Kvantify
Kvantify is a Danish company that develops quantum-accelerated molecular simulation software for drug discovery. It combines physics-based algorithms, high-performance cloud infrastructure, and quantum-ready tools to support teams in simulating molecular behavior faster.
Built on this platform, Koffee is a flagship product designed to predict binding affinity and unbinding kinetics in minutes. Using the FAST-VQE algorithm and supercomputers like Gefion, Koffee delivers rapid, simulation-ready outputs for drug candidates with minimal setup.
Key features include rapid calculations, API access to classical and quantum simulations, and the ability to model hundreds-of-qubit systems. These capabilities reduce experimental cycles and late-stage failure risk while supporting scalable drug development.
Kvantify raised EUR 10 million in seed funding to expand quantum algorithm development and chemistry-focused applications.
4. 3D-Printed Oral Medicines Move from Prototype to FDA Pipeline
3D-printing applications in pharma are moving from isolated prototypes to FDA-cleared drug candidates, hospital trials, and federally funded production lines.
For instance, the FDA approved two investigational new drug (IND) applications for 3D-printed oral capsules: T20G and T22. T20G is a gastric-retention anticoagulant, and T22 is a GLP-1 capsule – both developed by Triastek and cleared within 60 days of each other.
This pace signals that regulators now accept digital design files and novel geometries, much like traditional chemistry data.
Meanwhile, 3D printing is expanding beyond small molecules. In July 2024, BioNTech partnered with Triastek to co-develop oral RNA tablets, which is a leap into complex biologics delivered via customized pills. This collaboration underscores the platform’s versatility across therapeutic classes.
This growth is tied to real problems. 3D printing solves dose-flexibility gaps in pediatrics and geriatrics by enabling micro-tablets and easy-to-swallow formats, as shown in Spanish rare-disease wards.
It also eliminates waste from overstocking and empowers print-on-demand production during supply shocks.
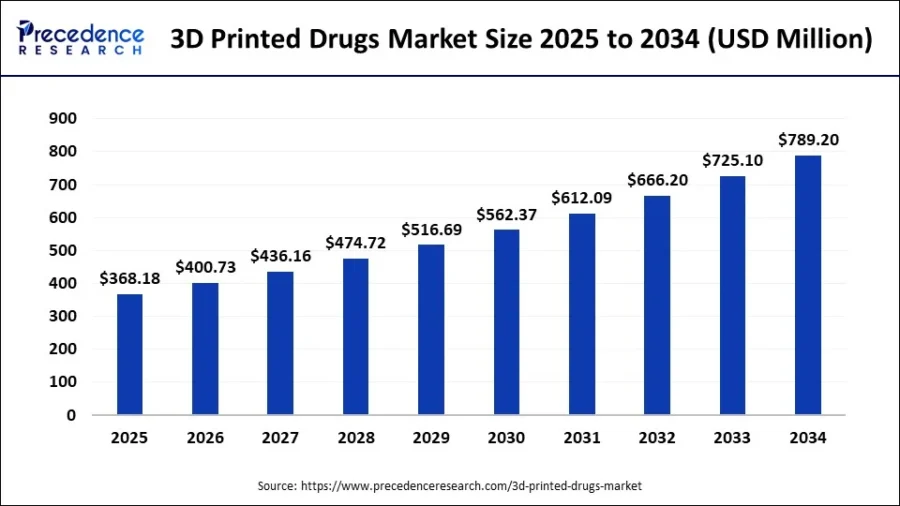
Credit: Precedence Research
Market forecasts estimate global revenue for 3D-printed drugs will nearly double from USD 368 million in 2025 to USD 789 million by 2034, supported by a 9% CAGR.
Funding momentum supports this trend. For example, Triastek has raised over USD 102 million. This includes a recent USD 20.4 million pre-C round to expand clinical manufacturing.
Moreover, serialization frameworks from DSCSA now enable tracking of geometry files. This makes “print-anywhere” production a regulatory reality.
Business Impacts & Opportunities: 3D-Printed Oral Medicines in Pharma
Faster Development Cycles, Faster Submissions
3D printing compresses formulation timelines with rapid, same-day tablet design and testing. This enables R&D teams to speed up iterations, adjust trial protocols, and regulatory submissions for complex oral therapies.
Personalized Medications Boost Adherence
Precision printing enables the customization of polypills according to individual patient needs, including dose, shape, and release profiles. This is beneficial for oncology, pediatrics, and geriatrics, where standard tablets often fall short on effectiveness and compliance.
Decentralized Production, No Cold Chain Required
On-demand tablet printing at hospitals or contract development and manufacturing organizations (CDMOs) reduces dependency on central factories, transport, and storage. This also reduces lead times and lowers the risk of stockouts, which is ideal for rare diseases or remote distribution.
Real-World Deployments & Pilots
FabRx’s M3DIMAKER enables Personalized Breast Cancer Polypills
Gustave Roussy initiated a clinical trial using personalized tamoxifen polypills produced with FabRx’s M3DIMAKER-GMP 3D printer. This marks the first use of GMP-compliant 3D printing in a European hospital pharmacy for a breast cancer study.
Aprecia’s ZipDose becomes First FDA-Approved 3D-Printed Drug Product
Aprecia’s ZipDose technology is used in the epilepsy medication Spritam. This became the first FDA-approved 3D-printed drug product due to its high-porosity formulation. Its rapid disintegration set a regulatory precedent for additive manufacturing in oral drug delivery.
Spotlighting an innovator: InkVivo Technologies
InkVivo Technologies is a Swiss startup that designs 3D-printed gastro-retentive capsules for oral drug delivery. The company uses fused deposition modeling (FDM) and biomaterials to create hollow capsular devices. These are then loaded with immediate-release tablets.
Each capsule floats in the stomach due to an internal air pocket. A fast-dissolving propellant seals off fluid-entry points and triggers a controlled release through engineered openings. By adjusting the number, size, and placement of these ports, the system personalizes drug release.
As a result, the company’s solution shortens formulation timelines and improves drug bioavailability for conditions like anemia and Type 2 diabetes.
5. Robotic Aseptic Fill-Finish: Reduces Contamination Risk and CMC Costs
Most sterile product recalls trace back to operator intervention, as human error is the top contamination risk. For instance, there’s a 37% contamination risk in sterile-to-sterile transfers.
To address this, regulators are pushing new sterility standards. For instance, the EU’s Annex 1 now favors closed, gloveless isolators as the risk control option for aseptic processing.
Pharma pipelines are also transitioning towards mRNA, CGT, and complex biologics. These demand faster changeovers and more flexible manufacturing setups. But maintaining round-the-clock staffing for fill-finish lines has become increasingly difficult worldwide.
Therefore, robotic fill-finish systems offer a solution. They operate in fully enclosed isolators without glove ports and eliminate human intervention from the critical zone to produce commercial-scale syringes and vials.
For instance, Selkirk Pharma launched ClinFAST, a fully robotic SKAN-pod system tailored for clinical-scale fill-finish. In February 2025, Selkirk announced successful media-fill trials. At the same time, SKAN raised its stake in Aseptic Technologies to 90%, strengthening its capabilities in high-demand robotic aseptic platforms.
Investments in next-generation filling lines, advanced robotics, and energy-efficient cleanrooms surpassed EUR 1.1 billion in the past two years.
Business Impact & Opportunities
Reduces Contamination Risk and Batch Failures
Fully automated, gloveless isolator systems lower human interventions in sterile zones. This reduces the risk of contamination and sterility breaches.
Accelerates Product Readiness and Tech Transfer
Modular robotic platforms simplify scale-up and validation across sites. This shortens CMC timelines, speeds regulatory filings, and supports faster launches for high-priority assets like oncology and mRNA-based products.
Enables Economic Small-Batch Production
Robotic systems offer high flexibility, which easily switches between vials, syringes, and cartridges. This supports low-volume, high-value production runs for orphan drugs, personalized medicine, and clinical batches.
Real-World Deployments & Pilots
White Raven becomes EU’s First GMP-Certified Site for SA25 Robotic Fill Line
White Raven CDMO announced that it received full GMP certification for Europe’s first commercial Cytiva SA25 robotic isolator. The approval was granted after a successful media fill and EU Annex 1 inspection.
WuXi Biologics expands Leverkusen Site with High-Capacity Prefilled Syringe Line
WuXi Biologics announced the addition of a new high-speed prefilled syringe line at its Leverkusen facility. It is designed to meet growing global demand for sterile, ready-to-administer biologics. The line will enhance the site’s drug product capabilities and offer clients flexible, scalable fill-finish solutions.
Novo Nordisk’s EUR 2.3 B Robotic Fill-Finish Expansion in Italy
Italy granted “national importance” status to Novo Nordisk’s EUR 2.3 billion fill-finish facility expansion in Anagni. The investment includes advanced robotic production lines to support its high-growth GLP-1 portfolio. The expansion will also improve sterile manufacturing capacity while aligning with the EU Annex 1 standards.
Spotlighting an innovator: Robotics Labs
Robotics Labs is a Dutch startup that develops robotic systems for aseptic fill-finish in sterile pharma environments. Its main products, InoBot and MicroBot, operate inside enclosed ISO-5 isolators using vision-guided Cartesian robotic arms.
These robots handle tasks like sample preparation, liquid or powder filling, stoppering, sealing, and automated plate or container exchange. The company offers modular platforms: InoBot for 3M Petrifilm plates and MicroBot for Petri dishes.
These modular platforms combine vision-guided robotics, gloveless isolators, and real-time process control to automate aseptic fill-finish workflows. They ensure full environmental control in ISO-5 cleanrooms, with GMP Annex 1 compliance built in.
These systems also work inside ISO-7 rooms to reduce infrastructure and monitoring demands. By removing human touchpoints and adding in-process controls, Robotics Labs reduces contamination risks and boosts yield.
6. Integrated Digital Twins Accelerate Scale-Up and Regulatory Readiness
Digital twins have evolved into full-scale platforms to support pharma factory operations.
For example, AstraZeneca’s Lighthouse plants deploy process twins for AI scheduling and predictive maintenance. These sites recorded 56% productivity gains and 67% reductions in development lead time for launching new products.
Pharma companies are also bringing digital twins into clinical development and real-world evidence (RWE).
For instance, Merck expanded its work with Unlearn to deploy synthetic patient twins and shorten immunology trial timelines, and reduce enrollment needs. Roche teamed up with CERN to model complex patients with multimorbidity twins.
Further, integrated digital twins offer end-to-end integrations. Plant twins now link to GMP data historians, while trial twins simulate full control arms. Pharma manufacturers using integrated twins are also able to reduce tech-transfer timelines, raise plant-wide efficiency, and accelerate market access by months.
With a 30.2% CAGR, the pharma manufacturing digital twins market will reach USD 8.5 billion by 2032. On the clinical side, the healthcare twin market is projected to reach USD 3.55 billion by 2030.
Business Impact & Opportunities
End-to-End Visibility Improves Operational Efficiency
Digital twins replicate real-world manufacturing lines and clinical workflows in real time. This allows pharma teams to simulate changes, optimize yield, and detect bottlenecks before they impact production.
Accelerated Scale-Up and Reduced Capital Spends
Standardized digital twin templates speed up tech transfer and reduce the need for revalidation across manufacturing sites. Therefore, the capital expenditure reduces and speeds up the rollout of new production lines. This is very effective for vaccines and next-gen biologics.
Transforms Clinical Trials with Synthetic Patient Models
Virtual patient twins reduce trial size, optimize protocol design, and accelerate recruitment. These in-silico tools lower costs, improve study predictability, and make trials more inclusive for hard-to-enroll disease areas.
Real-World Deployments & Pilots
Sanofi deploys Digital Twins to upgrade Trials and Manufacturing
Sanofi introduced a digital modeling and simulation accelerator aimed at modernizing clinical trials and manufacturing operations. The initiative integrates digital twin technology to simulate production lines and clinical workflows. This enabled outcome prediction and process optimization while reducing the risk of drug shortages.
Pfizer uses Process Digital Twins to enhance Bioreactor Performance
In order to model fluid dynamics inside its single-use bioreactors, Pfizer teamed up with M-Star CFD. This uncovered issues with mixing and gas transfer at a commercial scale. By using M-Star’s computational modeling platform, Pfizer identified non-uniform mixing challenges in their commercial-scale bioreactors. These insights led to design modifications and operational improvements that enhanced cell culture performance and process robustness.
Spotlighting an innovator: Blynksolve
Irish startup Blynksolve offers a no-code digital twin platform for pharma plant operations and in-silico clinical trial simulations.
The platform connects live plant data to its analytics module, Blynkinsights. This allows teams to visualize equipment performance, track operations, and monitor processes in real time.
It also includes a knowledge twin, Blynkdesign, for collaborative process design, tech transfer, MES planning, and risk assessments like HAZOP and FMEA. Blynksolve uses easy-to-integrate tools, AI-powered quality checks, and modular features to connect teams and streamline pharma workflows.
By centralizing data and workflows, the platform reduces errors and downtime while accelerating projects.
7. Smart Design & Continuous Manufacturing Lowers API Waste and Speeds Time-to-Market
Smart design and continuous manufacturing are driving speed, scale, and sustainability in pharmaceutical operations. Therefore, pharma leaders are making significant investments to stay aligned with the momentum.
For instance, Lilly raised USD 4.5 billion for “Medicine Foundry” in Indiana that merges R&D with advanced manufacturing for faster clinical turnaround.
Smart manufacturing isn’t confined to new builds. Johnson & Johnson Innovative Medicine is also investing EUR 125 million to retrofit its tablet plant with a continuous manufacturing oral-dose line. The upgrade is expected to expand capacity by more than 25%.
CDMOs are also joining the movement. In April 2025, Lonza partnered with the UK’s CMAC hub to scale continuous flow crystallization and solvent-efficient APIs. Their joint platform directly supports carbon reduction mandates and reduces material costs.
Meanwhile, Enzene Biosciences is building a fully continuous biologics site in New Jersey to expand end-to-end perfusion capacity for mAbs.
Moreover, governments are backing this shift. DARPA’s EQUIP-A-Pharma program, launched in May 2025, will co-fund four continuous pilot sites in the US, with a focus on validating digital release protocols. The move injects public-sector confidence into what was once a risk-heavy strategy.
Business Impact & Opportunities
Accelerates Launch Timelines and Cuts API Waste
Integrated continuous manufacturing lines optimize development lead times and reduce active ingredient usage. This speeds time-to-market while lowering material costs across the drug lifecycle.
Modular Designs reduce CapEx and speed up GMP readiness
Flexible skid-based systems allow installation in a less complex cleanroom environment. This is valuable for expanding production in cost-sensitive regions or niche drug volumes.
Enables High-Volume Biologics with Smaller Footprints
Continuous perfusion systems make it possible to scale biologics, such as mAbs and GLP-1s, within compact facilities. Therefore, it makes it possible for pharma companies to adapt quickly to demand spikes and makes biologics production more efficient and decentralized.
Real-World Deployments & Pilots
Merck Breaks Ground on USD 1 Billion Continuous Biologics Hub
Merck commenced construction of a USD 1 billion Biologics Center of Excellence in Wilmington, Delaware. The facility will feature fully integrated continuous perfusion suites and is intended to support scale-up for monoclonal antibody pipelines, including Keytruda biosimilars.
Kyowa Kirin designs a New Plant with Future-Ready Continuous Modules
Kyowa Kirin completed construction of a USD 118 million biologics facility in Japan. The plant is built “continuous-ready,” incorporating perfusion-based upstream processing and automated downstream purification systems. This allowed seamless scalability for future products without the need for batch revalidation.
Hovione’s GMP-ready ConsiGma CDC-Flex Line accelerates Early-Stage Tablet Production
Hovione’s continuous tableting line, ConsiGma CDC-Flex, has achieved full GMP certification at its Lisbon facility. This CDC platform enables rapid formulation and production of Phase I oral tablets. This allows clients to accelerate PK/tolerability studies and initial clinical trials. It also supports just-in-time (JIT) manufacturing to reduce development timelines and resource waste.
Spotlighting an innovator: Flownetics Engineering
Flownetics Engineering is an Indian startup that builds modular continuous manufacturing equipment for pharma and fine chemicals. Its systems combine 3D-printed flow reactor skids with AI-driven process tools and cloud-based control software.
The equipment supports manufacturing from lab to plant scale. It integrates hardware across lab, pilot, and production levels with smart software. Features include automated experiment design, predictive maintenance, and user-friendly interfaces for real-time process control.
Users also monitor and optimize chemical reactions throughout R&D, scale-up, and production. The equipment offers plug-and-play modular design as well as subscription-based factory-as-a-service (FaaS) and performance-as-a-service (PaaS) models. They reduce capital costs and simplify expansion.
8. Blockchain Traceability Improves Traceability and Cold Chain Compliance
Drug counterfeits are rising. For instance, China reports an increase in fake drugs by 22%. Moreover, the inability of traditional ERP systems to handle cell and gene therapy (CGT) cold chains forces pharma to adopt blockchain traceability.
For example, Merck and Zebra Technologies launched M-Trust, a cyber-physical platform, in March 2025. It links anti-counterfeit pigments to a Web3 ledger to meet the Drug Supply Chain Security Act (DSCSA) and forthcoming EU rules.
Also, Novo Nordisk put every Ozempic box in Thailand on Zuellig’s eZTracker. This blockchain QR is critical for high-demand GLP-1s, which lets patients verify packs in under two seconds.
Further, wholesalers are enforcing blockchain traceability. Cencora warned in 2025 that it would quarantine any shipment lacking EPCIS or ledger data.
At AUTOMA+ Zurich, PharmaLedger showed open-source ePI and “product-trust” layers that attach carbon and quality metadata to every unit. This laid the groundwork for ESG-linked passports.
Blockchain is also powering personalized medicine. Roche’s SAP-based CGT orchestration tracks chain-of-identity and cryo-logistics and earned consecutive SAP Innovation Awards.
Behind these developments is a surge in capital. Analysts now expect the blockchain market for the pharma supply chain to reach over USD 5.8 billion by 2030, with a CAGR exceeding 21%.
Business Impact & Opportunities
Counterfeit Protection builds Patient Trust
Blockchain-verified packaging allows instant product authentication at the point of use. This reduces the risk of counterfeits entering the supply chain. It also strengthens pharma companies’ credibility, especially in vulnerable or high-growth markets.
Safer Cold Chains for Advanced Therapies
In cell and gene therapy, where timing and temperature are critical, blockchain enables immutable handoff records and precise chain-of-custody logs. They build confidence among regulators, providers, and payers while supporting future personalized medicine models.
Faster Reconciliation, Leaner Inventory
Blockchain networks simplify batch-level traceability and reduce manual reconciliation across complex supply chains. This reduces the need for safety stock and frees up working capital.
Real-World Deployments & Pilots
DHL acquires CRYOPDP to boost its Blockchain-enabled CGT Cold Chain Network
In March 2025, DHL acquired CRYOPDP, a specialty courier handling 600 000+ biologic shipments annually. The move embeds blockchain-ready traceability into one of the largest global cell and gene therapy (CGT) networks. This laid the foundation for direct-to-patient delivery models with verified, tamper-evident custody.
EUR 2 Billion Blockchain-ready Investment in DHL Pharma Hubs
DHL announced in April 2025 that it will invest EUR 2 billion in its GDP-compliant pharma hubs by 2030. 50% of which is earmarked for North America. The investment signals a long-term commitment to integrating blockchain and IoT technologies, which aim to improve transparency and ensure the safe transport of temperature-sensitive therapies in regulated markets.
Spotlighting an innovator: TrueCold
TrueCold is a Spanish startup that offers a blockchain-powered platform to track pharma cold chain shipments from factories to patients. It collects temperature, location, and sensor data from smart trackers in real time.
All data is logged on a distributed ledger for tamper-proof and compliant data storage. The platform utilizes AI to detect anomalies, send alerts, and automate quality release steps.
TrueCold combines separate datasets into one system to make it ready for audits and meet ISO 27001, SOC 2, and FDA 21 CFR Part 11 standards. This avoids cold chain failures and speeds up clinical trial validations.
The platform also reduces the risk of temperature excursions, accelerates product release, and supports regulatory compliance and sustainability. It delivers a zero-waste cold chain intelligence layer to ensure that temperature-sensitive therapies reach patients safely.
9. Net-Zero & Low-Carbon Operations Become Strategic Differentiators
Pharmaceutical leaders have started the large-scale execution of net-zero and low-carbon operations.
In early 2025, AstraZeneca launched a biomethane plant in the UK that alone meets about 20% of the company’s global gas demand. At the same time, four major industry players (GSK, Haleon, Thermo Fisher, and Gilead) executed a combined purchase of 245 GWh/year of renewable energy for a 10-year term.
Decarbonization is also stretching into the supply chain. For instance, AstraZeneca and GSK’s China renewable power program aggregates 225 GWh of clean power for suppliers and avoids 250 000 tonnes of CO2 emissions per year.
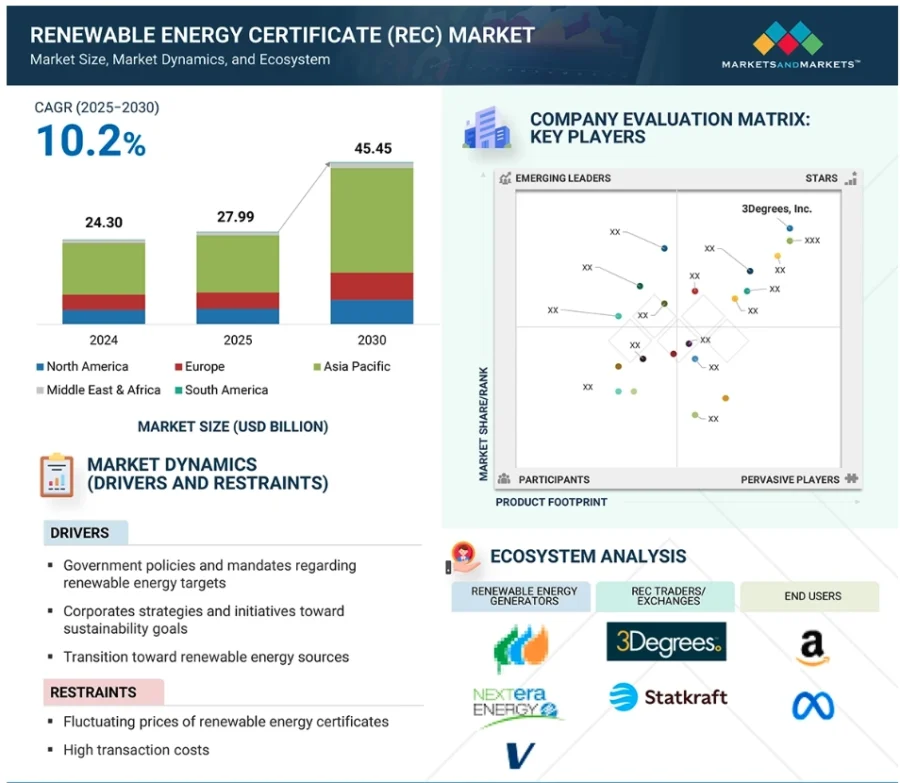
Credit: Marketsndmarkets
Moreover, the renewable energy certificate (REC) market is projected to reach USD 45.45 billion by 2030, while the global renewable energy market is expected to reach USD 4.86 trillion by 2033.
Meanwhile, 53% of pharma and biotech companies by revenue are now linked to UN race-to-zero commitments.
Business Impact & Opportunities
Energy Cost Stability through Renewable PPAs
Long-term renewable energy deals are supporting pharma manufacturers in stabilizing electricity costs. This is critical for energy-intensive operations like cleanroom HVAC, cold storage, and 24/7 fill-finish lines, where power volatility disrupts budgets and compliance.
Decarbonization as a Hedge Against Carbon Pricing
Shifting to onsite renewable heat (like biomethane, heat pumps, or solar thermal) offers pharma plants a path to reduce Scope-1 emissions without public subsidies. This avoids future carbon levies and improves access to sustainability-linked financing.
Carbon-Neutral Facilities Accelerate Regulatory Approvals and Incentives
New builds designed with low-emission infrastructure, like heat recovery, electric steam systems, or rooftop solar, are being prioritized for fast-track permitting in several markets. This shortens time-to-approval for new GMP capacity and often unlocks regional tax breaks.
Real-World Deployments & Pilots
Novartis breaks ground on Net-Zero BioCampus in Slovenia
In April 2025, Novartis began construction on a high-tech BioCampus in Menges, which is designed to run entirely carbon-neutral. The site combines rooftop solar, waste heat recovery, and electrified infrastructure. The facility serves as the company’s European pilot for net-zero biologics plants.
Takeda switches to Biomass-based Steam Plant at Singen site
Takeda switched on a biomass-powered steam plant at its German Singen facility, along with other measures to reduce 4000 to 6000 metric tons of CO2 emissions. It’s the first full-scale biomass conversion in Takeda’s global network.
Spotlighting an innovator: 4BlueTech
4BlueTech is a Spanish startup that leverages AI to optimize microalgae-based carbon capture systems. Its photobioreactors cultivate microalgae that absorb CO2 from the environment. AI algorithms optimize growth conditions by adjusting light, CO2 levels, and other environmental factors in real time to maximize carbon uptake.
The company’s offerings include traceable, IoT-enabled carbon capture-as-a-service (CaaS). The solution also works with wastewater to reduce freshwater use.
10. Agile & Convergent Regulation Unlocks Early Market Access
Agencies in the US, UK, and EU have started to include real-time reviews, cross-border recognition, and AI-ready evidence frameworks into practice. These tools enable faster approvals and leaner trials
For example, AbbVie’s Emrelis, an antibody drug conjugate (ADC) for lung cancer, secured FDA approval roughly seven months after submission under the real-time oncology review (RTOR) program.
Meanwhile, the UK’s new International Recognition Procedure (IRP) enabled a new drug to reach the market in just 30 days by accepting FDA reviews.
Regulators are also embracing new evidence models. The FDA’s January 2025 guidance formally allows AI-generated insights and digital twin simulations in submission packages. Sponsors now have a rulebook for including model-based predictions and synthetic control arms in trials.
This guidance leads to platform-level approvals. In April 2025, Cellares’ robotic Cell Shuttle became the first to receive the FDA’s Advanced Manufacturing Technology (AMT) designation. That means every therapy made on the platform gets fast-track regulatory access, thus reducing chemistry, manufacturing, and control (CMC) costs.
Regulatory systems are also being rewired for traceability. The US DSCSA mandates package-level serialization. 26 070 companies have enabled DSCSA compliance on TraceLink and created over 47 000 links to exchange EPCIS information.
The European Medicines Agency (EMA) is also scaling its DARWIN EU network to run 140 real-world data (RWD) studies annually. These data streams feed live safety and effectiveness updates into label revisions for continuous post-market monitoring.
Business Impact & Opportunities
Regulatory Acceleration Unlocks 6 to 12 Months of Extra Market Exclusivity
Regulatory programs like RTOR enabled AbbVie’s Emrelis to clear FDA review within a few months after NDA submission. This proves that rolling or parallel reviews fast-track launches and generate hundreds of millions in earlier revenue.
AI-Credibility Guidance Opens Path for Digital Evidence Submissions
The FDA’s January 2025 guidance introduces risk-tiered validation for AI and digital twin-generated evidence in IND/NDA filings. This enabled sponsors to compress clinical timelines using in silico models and real-world evidence.
Decentralized Trial Rules Reduce Enrollment Timelines and Costs
FDA’s final guidance allows remote assessments and wearable integration to offer lean-trial sponsors a green light to lower site overheads and boost participation in underrepresented geographies.
Real-World Deployments & Pilots
Johnson & Johnson extends RTOR to Drug-Device Combinations
The FDA accepted J&J’s NDA for TAR-200, an intravesical bladder-cancer therapy, under RTOR. The case expands real-time regulatory review to drug-device combination products. This decision opened the door for complex modalities to benefit from faster turnaround and iterative feedback loops.
Pfizer pilots Remote Evidence Models under FDA’s DCT Guidance
Pfizer’s 2025 “Clinical Trial Anywhere” initiative is set to reduce in-person visits by half through using decentralized clinical trial (DCT) frameworks aligned with new FDA guidance. The program combines digital consent, remote diagnostics, and cloud data exchange to enable hybrid trial formats that scale globally.
Spotlighting an innovator: Artos AI
Artos AI is a US-based startup that builds a regulatory intelligence platform powered by generative AI. It converts raw, unstructured data into submission-ready regulatory documents in minutes. The platform aggregates and analyzes information from internal company repositories and external databases, including FDA and other publicly available regulatory sources.
Using AI models, the platform drafts key regulatory filings such as INDs, NDAs, and BLAs. Built-in features like Inconsistency Intelligence track data dependencies and flag sections that need updates when inputs change, while the Source Tracer ensures all citations are audit-ready. This allows regulatory teams to reduce the time to prepare submission packages.
Explore the Latest Pharma Technologies & Companies to Stay Ahead
With thousands of emerging technologies shaping the future of pharma, identifying investment and partnership opportunities that deliver rapid returns remains a major challenge.
With access to over 7 million emerging companies and 20K+ technologies & trends globally, our AI and Big Data-powered Discovery Platform equips you with the actionable insights you need to stay ahead of the curve in your market.
Leverage this powerful tool to spot the next big thing before it goes mainstream. Stay relevant, resilient, and ready for what is next.
![Future of Pharma: 10 Trends Powering the Next Wave [2026-2030]](https://www.startus-insights.com/wp-content/uploads/2025/07/Future-of-Pharma-SharedImg-StartUs-Insights-noresize-1-420x236.webp)








