Our Innovation Analysts recently looked into emerging technologies and up-and-coming startups working on solutions for the pharma sector. As there is a large number of startups working on a wide variety of solutions, we decided to share our insights with you. This time, we are taking a look at 5 promising sexually transmitted disease (STD) solutions.
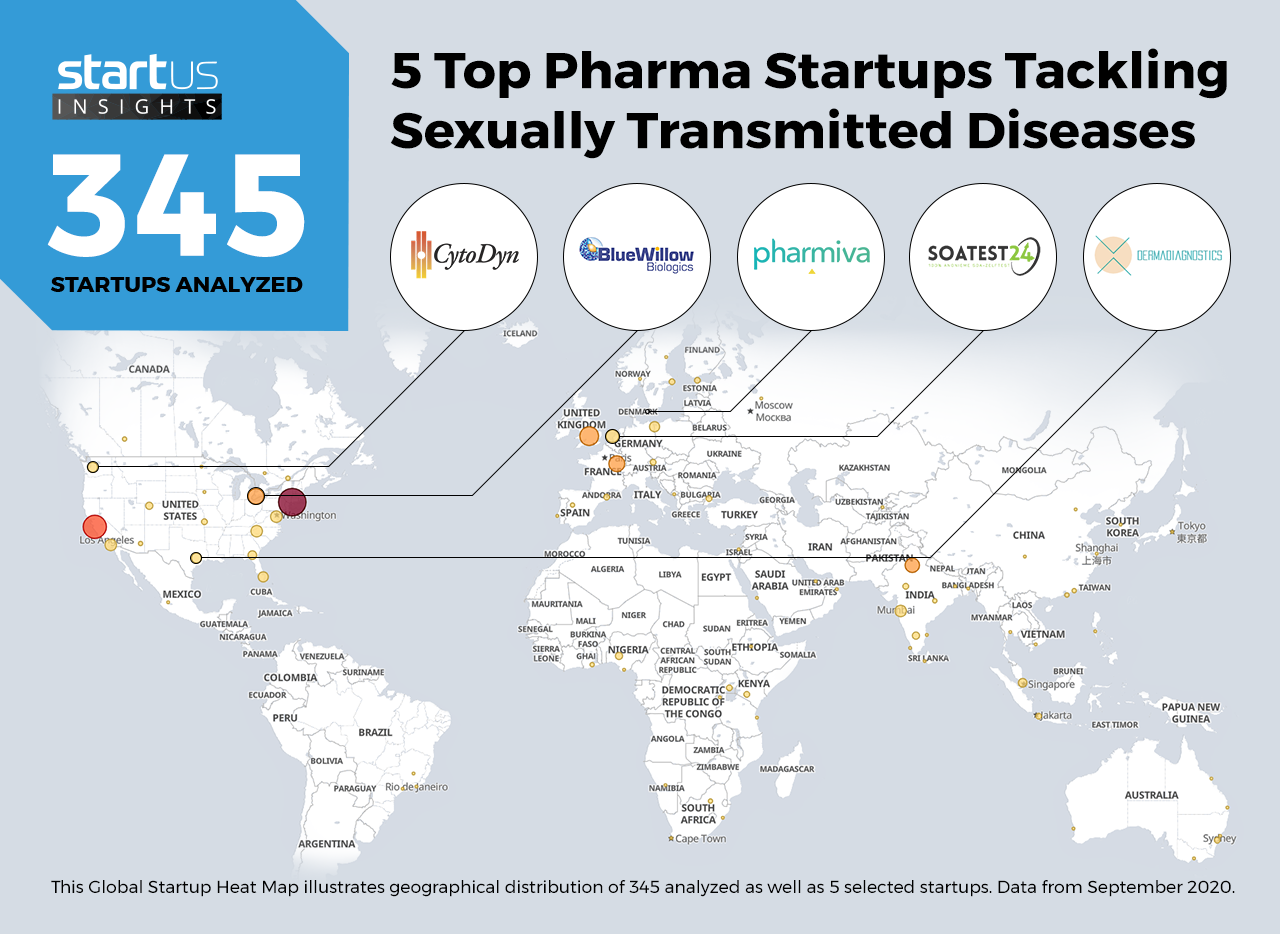
Heat Map: 5 Top Startups Tackling Sexually Transmitted Diseases
Using our StartUs Insights Platform, covering 1.116.000+ startups & emerging companies, we looked at innovation in the field of pharma. For this research, we identified 345 relevant solutions and picked 5 to showcase below. These companies were chosen based on a data-driven startup scouting approach, taking into account factors such as location, founding year, and relevance of technology, among others. Depending on your specific criteria, the top picks might look entirely different.
The Global Startup Heat Map below highlights 5 startups & emerging companies developing novel solutions to combat sexually transmitted diseases. Moreover, the Heat Map reveals regions that observe a high startup activity and illustrates the geographic distribution of all 345 companies we analyzed for this specific topic.
Click to download
dermadiagnostics – Non-Invasive Diagnostic Patch
One of the main reasons why people do not get regularly tested for STDs is due to the invasive nature of the test. Some sexually transmitted infections (STIs) are contagious and show symptoms in some patients many years after entering the body. Given the importance of controlling infectious diseases for public health, emerging pharma startups are working to develop non-invasive testing solutions for STDs.
The US-based startup dermadiagnostics provides a non-invasive dermal patch platform for healthcare facilities to use in diagnosing sexually transmitted infections, as well as cervical cancer. This diagnostic solution utilizes a microneedle-mediated intradermal diagnostic patch that detects asymptomatic STIs and gynecologic cancers. The startup’s products allow doctors and healthcare workers to appropriately test and provide the results on the same day. dermadiagnostics also provides patient-centered diagnosing in the area of gynecologic health.
SOA-Test – At-Home STI Diagnostics
Filing official paperwork and a reluctance to openly discuss sexual activity further deters people, especially younger cohorts, from getting tested for STIs. This encourages emerging diagnostics companies to develop novel home-based testing solutions for STDs. Startups utilize secure cloud-based networks to collect, analyze, and provide results to users.
Dutch HealthTech startup SOA-Test offers an online STI test that is easy-to-use and discreet. Users get to compare and order a suitable STI test. Then, users read the STI test documents and collect their urine samples. In just ten minutes, the startup enables the test to provide the results, allowing users to make confident decisions about their sexual health. SOA-Test covers STDs such as chlamydia, human immunodeficiency virus (HIV), Hepatitis C, and human papillomavirus (HPV), among others.
Pharmiva – Antibiotics-Free Bacterial Vaginosis Treatment
Millions of women around the world suffer from bacterial vaginosis, an overgrowth of, in some cases harmful, bacteria. This causes imbalances in vaginal discharge, causes itching or burning in the vagina, and may also lead to contraction of STIs. Further, many women with this condition show no symptoms. This encourages startups working on women’s health products to develop suitable solutions to alleviate vaginal health.
Swedish startup Pharmiva is utilizing its patented local drug delivery system Venerol, a thermoactivated formulation, based on naturally occurring substances and crystalline lipids. Venerol works to stabilize both hydrophilic and hydrophobic compounds that may otherwise be subject to degradation. The startup’s first medical device product, a vaginal mousse, activates just below body temperature, coats the mucosa, and slowly releases the compounds upon application.
BlueWillow Biologics – Herpes Simplex Virus-2 (HSV-2) Vaccine
HSV-2 is a highly contagious infection that affects almost half a billion people globally. In many cases, carriers tend to be asymptomatic and can be detected only with regular testing. HSV-2, in particular, is caused by sexual intercourse and also affects the genitals. Further, people suffering from HIV may face life-threatening complications if they also contract herpes simplex viruses. Given the scale of the infection prevalence, pharma companies are working to find effective vaccines for both types of HSV.
The US-based startup BlueWillow Biologics is currently developing an intranasal nanoemulsion (NE) vaccine that offers protection against HSV-2. The vaccine allows for both a systemic and mucosal immune response. The mucosal immunity elicited by NE vaccines provides critical protection against STIs at their point of entry. The startup is planning Phase 1 human clinical trials to advance both its prophylactic and therapeutic NE vaccine candidates.
CytoDyn – Antiviral Agents For HIV
Despite recent advancements in the availability of lifelong antiretroviral therapy (ART) for HIV autoimmune deficiency syndrome (AIDS), several people still cannot access suitable treatment. Current medication for HIV/AIDS works to prevent viral transmission while helping individual immune systems. With increasing investments, emerging pharma startups are developing novel solutions to fight, and eventually eliminate, HIV/AIDS.
The US-based startup Cytodyn is developing Leronlimab (PRO 140), a new class of HIV/AIDS antiviral agents that protect healthy cells from viral infection. This self-injectable, subcutaneous injection for HIV offers advantages over the current treatments that include no serious side effects and serious adverse events (SAEs) related to Leronlimab. In addition, this solution enables enhanced compliance and increases its half-life. Leronlimab is currently awaiting US Food and Drug Administration (FDA) approval, after completing Phase 3 clinical trials.
What About The Other 340 Solutions?
While we believe data is key to creating insights it can be easy to be overwhelmed by it. Our ambition is to create a comprehensive overview and provide actionable innovation intelligence that enables you to achieve your goals faster. The 5 solutions tackling sexually transmitted diseases showcased above are promising examples out of 345 we analyzed for this article. To identify the most relevant solutions based on your specific criteria, get in touch.